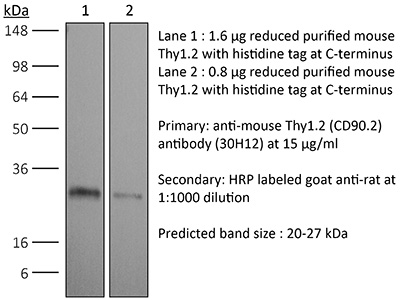
InVivoPlus anti-mouse Thy1.2 (CD90.2)
| Clone | 30H12 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalog # | BP0066 | ||||||||||
| Category | InVivoPlus Antibodies | ||||||||||
| Price |
|
The 30H12 monoclonal antibody reacts with mouse Thy1.2 also known as CD90.2. Thy1.2 is expressed by thymocytes and mature T lymphocytes as well as hematopoietic stem cells, neurons, epithelial cells, and fibroblasts. Thy1.2 is expressed only by certain mouse strains including C57BL/6, BALB/c, CBA, C3H, C58/, SJL, DBA, and NZB/. Thy1.2 is a 25-35 kDa GPI-anchored membrane glycoprotein and a member of the immunoglobulin superfamily. The function of Thy1.2 has not been fully elucidated but is thought to play roles in cognition, axon growth, T lymphocyte function, and apoptosis. The 30H12 monoclonal antibody has been reported to induce Ca2+ flux in thymocytes. This antibody is particularly useful for depletion of T lymphocytes.
| Isotype | Rat IgG2b, κ |
| Recommended Isotype Control(s) | InVivoPlus™ rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure™ pH 7.0 Dilution Buffer |
| Immunogen | Mouse thymus or spleen |
| Reported Applications |
|
| Formulation |
|
| Endotoxin |
|
| Aggregation |
|
| Purity |
|
| Sterility | 0.2 μM filtered |
| Production | Purified from tissue culture supernatant in an animal free facility |
| Purification | Protein G |
| RRID | AB_1107682 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Test Results |
|
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
INVIVOPLUS ANTI-MOUSE THY1.2 (CD90.2) (CLONE: 30H12)
Seo, G. Y., et al. (2018). “LIGHT-HVEM Signaling in Innate Lymphoid Cell Subsets Protects Against Enteric Bacterial Infection.” Cell Host Microbe 24(2): 249-260 e244. PubMed
Innate lymphoid cells (ILCs) are important regulators of early infection at mucosal barriers. ILCs are divided into three groups based on expression profiles, and are activated by cytokines and neuropeptides. Yet, it remains unknown if ILCs integrate other signals in providing protection. We show that signaling through herpes virus entry mediator (HVEM), a member of the tumor necrosis factor (TNF) receptor superfamily, in ILC3 is important for host defense against oral infection with the bacterial pathogen Yersinia enterocolitica. HVEM stimulates protective interferon-gamma (IFN-gamma) secretion from ILCs, and mice with HVEM-deficient ILC3 exhibit reduced IFN-gamma production, higher bacterial burdens and increased mortality. In addition, IFN-gamma production is critical as adoptive transfer of wild-type but not IFN-gamma-deficient ILC3 can restore protection to mice lacking ILCs. We identify the TNF superfamily member, LIGHT, as the ligand inducing HVEM signals in ILCs. Thus HVEM signaling mediated by LIGHT plays a critical role in regulating ILC3-derived IFN-gamma production for protection following infection.
Bouchery, T., et al. (2015). “ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms.” Nat Commun 6: 6970. PubMed
Defining the immune mechanisms underlying protective immunity to helminth infection remains an important challenge. Here we report that lung CD4(+) T cells and Group 2 innate lymphoid cells (ILC2s) work in concert to block Nippostrongylus brasiliensis (Nb) development in the parenchyma within 48 h in mice. Immune-damaged larvae have a striking morphological defect that is dependent on the expansion of IL-13-producing ILC2 and CD4(+) T cells, and the activation of M2 macrophages. This T-cell requirement can be bypassed by administration of IL-2 or IL-33, resulting in expansion of IL-13-producing ILC2s and larval killing. Depletion of ILC2s inhibits larval killing in IL-2-treated mice. Our results broaden understanding of ILC2’s role in immunity to helminths by demonstrating that they not only act as alarmin sensors, but can also be sustained by CD4(+) T cells, ensuring both the prompt activation and the maintenance of IL-13-dependent M2 macrophage immunity in the lung.
Brasseit, J., et al. (2015). “CD4 T cells are required for both development and maintenance of disease in a new mouse model of reversible colitis.” Mucosal Immunol. doi: 10.1038/mi.2015.93. PubMed
Current therapies to treat inflammatory bowel diseases have limited efficacy, significant side effects, and often wane over time. Little is known about the cellular and molecular mechanisms operative in the process of mucosal healing from colitis. To study such events, we developed a new model of reversible colitis in which adoptive transfer of CD4+CD45RBhi T cells into Helicobacter typhlonius-colonized lymphopenic mice resulted in a rapid onset of colonic inflammation that was reversible through depletion of colitogenic T cells. Remission was associated with an improved clinical and histopathological score, reduced immune cell infiltration to the intestinal mucosa, altered intestinal gene expression profiles, regeneration of the colonic mucus layer, and the restoration of epithelial barrier integrity. Notably, colitogenic T cells were not only critical for induction of colitis but also for maintenance of disease. Depletion of colitogenic T cells resulted in a rapid drop in tumor necrosis factor alpha (TNFalpha) levels associated with reduced infiltration of inflammatory immune cells to sites of inflammation. Although neutralization of TNFalpha prevented the onset of colitis, anti-TNFalpha treatment of mice with established disease failed to resolve colonic inflammation. Collectively, this new model of reversible colitis provides an important research tool to study the dynamics of mucosal healing in chronic intestinal remitting-relapsing disorders.
Finkin, S., et al. (2015). “Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma.” Nat Immunol. doi: 10.1038/ni.3290. PubMed
Ectopic lymphoid-like structures (ELSs) are often observed in cancer, yet their function is obscure. Although ELSs signify good prognosis in certain malignancies, we found that hepatic ELSs indicated poor prognosis for hepatocellular carcinoma (HCC). We studied an HCC mouse model that displayed abundant ELSs and found that they constituted immunopathological microniches wherein malignant hepatocyte progenitor cells appeared and thrived in a complex cellular and cytokine milieu until gaining self-sufficiency. The egress of progenitor cells and tumor formation were associated with the autocrine production of cytokines previously provided by the niche. ELSs developed via cooperation between the innate immune system and adaptive immune system, an event facilitated by activation of the transcription factor NF-kappaB and abolished by depletion of T cells. Such aberrant immunological foci might represent new targets for cancer therapy.
Wilson, K. A., et al. (2015). “Depletion of B220NK1.1 cells enhances the rejection of established melanoma by tumor-specific CD4 T cells.” Oncoimmunology 4(8): e1019196. PubMed
Five-year survival rates for patients diagnosed with metastatic melanoma are less than 5%. Adoptive cell transfer (ACT) has achieved an objective response of 50% by Response Evaluation Criteria in Solid Tumors (RECIST) in this patient population. For ACT to be maximally effective, the host must first be lymphodepleted. It is hypothesized that lymphodepletion may remove regulatory elements and cytokine sinks, or increase the activation and availability of antigen presenting cells (APCs). We use an in vivo model to study the ACT of tumor-associated antigen (TAA)-specific CD4+ T cells (TRP-1 cells). We have discovered that depletion of NK1.1+ cells enhances the rejection of established melanoma tumors by adoptively transferred TRP-1 CD4+ T cells. NK1.1+ cell depletion increases the number of CD4+ T cells, the serum concentration of pro-inflammatory cytokines, autoimmune vitiligo, host survival and prevented recurrence after ACT. Because multiple cells express NK1.1, we targeted different NK1.1+ cell populations using antibodies specific for NK cells, pre-mNK cells, and innate lymphoid cells (ILCs). Our data suggests that NK1.1+B220+ pre-mNK cells (also known as interferon-producing killer dendritic cells; IKDCs) are an important inhibitor of the CD4+ T cell response to melanoma. Understanding this mechanism may help design new immunotherapies to modulate the activity of pre-mNKs in the face of an antitumor immune response and inhibit their suppression of adoptively transferred T cells.
Yang, Q., et al. (2015). “Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice.” J Allergy Clin Immunol. PubMed
BACKGROUND: Asthmatic patients are highly susceptible to air pollution and in particular to the effects of ozone (O3) inhalation, but the underlying mechanisms remain unclear. OBJECTIVE: Using mouse models of O3-induced airway inflammation and airway hyperresponsiveness (AHR), we sought to investigate the role of the recently discovered group 2 innate lymphoid cells (ILC2s). METHODS: C57BL/6 and BALB/c mice were exposed to Aspergillus fumigatus, O3, or both (3 ppm for 2 hours). ILC2s were isolated by means of fluorescence-activated cell sorting and studied for Il5 and Il13 mRNA expression. ILC2s were depleted with anti-Thy1.2 mAb and replaced by means of intratracheal transfer of ex vivo expanded Thy1.1 ILC2s. Cytokine levels (ELISA and quantitative PCR), inflammatory cell profile, and AHR (flexiVent) were assessed in the mice. RESULTS: In addition to neutrophil influx, O3 inhalation elicited the appearance of eosinophils and IL-5 in the airways of BALB/c but not C57BL/6 mice. Although O3-induced expression of IL-33, a known activator of ILC2s, in the lung was similar between these strains, isolated pulmonary ILC2s from O3-exposed BALB/c mice had significantly greater Il5 and Il13 mRNA expression than C57BL/6 mice. This suggested that an altered ILC2 function in BALB/c mice might mediate the increased O3 responsiveness. Indeed, anti-Thy1.2 treatment abolished but ILC2s added back dramatically enhanced O3-induced AHR. CONCLUSIONS: O3-induced activation of pulmonary ILC2s was necessary and sufficient to mediate asthma-like changes in BALB/c mice. This previously unrecognized role of ILC2s might help explain the heightened susceptibility of human asthmatic airways to O3 exposure.
Deshmukh, H. S., et al. (2014). “The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice.” Nat Med 20(5): 524-530. PubMed
Neonatal colonization by microbes, which begins immediately after birth, is influenced by gestational age and the mother’s microbiota and is modified by exposure to antibiotics. In neonates, prolonged duration of antibiotic therapy is associated with increased risk of late-onset sepsis (LOS), a disorder controlled by neutrophils. A role for the microbiota in regulating neutrophil development and susceptibility to sepsis in the neonate remains unclear. We exposed pregnant mouse dams to antibiotics in drinking water to limit transfer of maternal microbes to the neonates. Antibiotic exposure of dams decreased the total number and composition of microbes in the intestine of the neonates. This was associated with decreased numbers of circulating and bone marrow neutrophils and granulocyte/macrophage-restricted progenitor cells in the bone marrow of antibiotic-treated and germ-free neonates. Antibiotic exposure of dams reduced the number of interleukin-17 (IL-17)-producing cells in the intestine and production of granulocyte colony-stimulating factor (G-CSF). Granulocytopenia was associated with impaired host defense and increased susceptibility to Escherichia coli K1 and Klebsiella pneumoniae sepsis in antibiotic-treated neonates, which could be partially reversed by administration of G-CSF. Transfer of a normal microbiota into antibiotic-treated neonates induced IL-17 production by group 3 innate lymphoid cells (ILCs) in the intestine, increasing plasma G-CSF levels and neutrophil numbers in a Toll-like receptor 4 (TLR4)- and myeloid differentiation factor 88 (MyD88)-dependent manner and restored IL-17-dependent resistance to sepsis. Specific depletion of ILCs prevented IL-17- and G-CSF-dependent granulocytosis and resistance to sepsis. These data support a role for the intestinal microbiota in regulation of granulocytosis, neutrophil homeostasis and host resistance to sepsis in neonates.
Ermann, J., et al. (2014). “Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet-/-.Rag2-/- (TRUC) mice.” Proc Natl Acad Sci U S A 111(25): E2559-2566. PubMed
T-bet(-/-).Rag2(-/-) (TRUC) mice spontaneously develop microbiota-driven, TNF-mediated large bowel inflammation that resembles human ulcerative colitis. We show here that IL-23 and IL-1-dependent secretion of IL-17A by innate lymphoid cells (ILCs; defined as CD45(+)lin(-)Thy1(hi)NKp46(-)) is a second critical pathway in this model. Using an in vitro coculture system of bone marrow-derived dendritic cells (DCs) and freshly isolated FACS-purified ILCs, we demonstrate that IL-23 and IL-1 secreted by DCs in response to microbial stimulation work together to induce IL-17A production by ILCs. TNF is not required for IL-17A secretion by ILCs in vitro but synergizes with IL-17A to induce the expression of neutrophil-attracting chemokines. Upstream, activation of the IL-23/IL-17A axis is regulated by nucleotide-binding oligomerization domain containing (Nod)/receptor-interacting serine-threonine kinase 2 (Ripk2) signals in DCs. Genetic ablation of the Nod/Ripk2 signaling pathway protects TRUC mice from developing colitis without affecting the colitogenicity of the intestinal microbiota. Our data provide insight into the complex network of interactions between IL-17A-secreting ILCs and other components of the innate immune system in the development of colitis.
Engelmann, S., et al. (2013). “T cell-independent modulation of experimental autoimmune encephalomyelitis in ADAP-deficient mice.” J Immunol 191(10): 4950-4959. PubMed
The adhesion- and degranulation-promoting adaptor protein (ADAP), expressed in T cells, myeloid cells, and platelets, is known to regulate receptor-mediated inside-out signaling leading to integrin activation and adhesion. In this study, we demonstrate that, upon induction of active experimental autoimmune encephalomyelitis (EAE) by immunization with the myelin oligodendrocyte glycoprotein35-55 peptide, ADAP-deficient mice developed a significantly milder clinical course of EAE and showed markedly less inflammatory infiltrates in the CNS than wild-type mice. Moreover, ADAP-deficient recipients failed to induce EAE after adoptive transfer of myelin oligodendrocyte glycoprotein-specific TCR-transgenic T cells (2D2 T cells). In addition, ex vivo fully activated 2D2 T cells induced significantly less severe EAE in ADAP-deficient recipients. The ameliorated disease in the absence of ADAP was not due to expansion or deletion of a particular T cell subset but rather because of a strong reduction of all inflammatory leukocyte populations invading the CNS. Monitoring the adoptively transferred 2D2 T cells over time demonstrated that they accumulated within the lymph nodes of ADAP-deficient hosts. Importantly, transfer of complete wild-type bone marrow or even bone marrow of 2D2 TCR-transgenic mice was unable to reconstitute EAE in the ADAP-deficient animals, indicating that the milder EAE was dependent on (a) radio-resistant nonhematopoietic cell population(s). Two-photon microscopy of lymph node explants revealed that adoptively transferred lymphocytes accumulated at lymphatic vessels in the lymph nodes of ADAP-deficient mice. Thus, our data identify a T cell-independent mechanism of EAE modulation in ADAP-deficient mice.
Gladiator, A., et al. (2013). “Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection.” J Immunol 190(2): 521-525. PubMed
IL-17-mediated immunity has emerged as a crucial host defense mechanism against fungal infections. Although Th cells are generally thought to act as the major source of IL-17 in response to Candida albicans, we show that fungal control is mediated by IL-17-secreting innate lymphoid cells (ILCs) and not by Th17 cells. By using a mouse model of oropharyngeal candidiasis we found that IL-17A and IL-17F, which are both crucial for pathogen clearance, are produced promptly upon infection in an IL-23-dependent manner, and that ILCs in the oral mucosa are the main source for these cytokines. Ab-mediated depletion of ILCs in RAG1-deficient mice or ILC deficiency in retinoic acid-related orphan receptor c(-/-) mice resulted in a complete failure to control the infection. Taken together, our data uncover the cellular basis for the IL-23/IL-17 axis, which acts right at the onset of infection when it is most needed for fungal control and host protection.
McHedlidze, T., et al. (2013). “Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis.” Immunity 39(2): 357-371. PubMed
Liver fibrosis is a consequence of chronic liver diseases and thus a major cause of mortality and morbidity. Clinical evidence and animal studies suggest that local tissue homeostasis is disturbed due to immunological responses to chronic hepatocellular stress. Poorly defined stress-associated inflammatory networks are thought to mediate gradual accumulation of extracellular-matrix components, ultimately leading to fibrosis and liver failure. Here we have reported that hepatic expression of interleukin-33 (IL-33) was both required and sufficient for severe hepatic fibrosis in vivo. We have demonstrated that IL-33’s profibrotic effects related to activation and expansion of liver resident innate lymphoid cells (ILC2). We identified ILC2-derived IL-13, acting through type-II IL-4 receptor-dependent signaling via the transcription factor STAT6 and hepatic stellate-cell activation, as a critical downstream cytokine of IL-33-dependent pathologic tissue remodeling and fibrosis. Our data reveal key immunological networks implicated in hepatic fibrosis and support the concept of modulation of IL-33 bioactivity for therapeutic purposes.
Freeman, M. L., et al. (2012). “gamma-Herpesvirus reactivation differentially stimulates epitope-specific CD8 T cell responses.” J Immunol 188(8): 3812-3819. PubMed
The gamma-herpesviruses are characterized by their ability to establish lifelong latency. Subsequent immune suppression leads to viral reactivation from latency and the onset of a variety of pathologies, including lymphoproliferative disease and cancers. CD8 T cells play a key role in preventing reactivation of latent virus. Therefore, to develop effective therapeutic immune strategies, it is essential to understand the maintenance of CD8 T cell responses during latency. Because the gamma-herpesviruses are highly species-specific and mice cannot be infected with the human pathogens, EBV or Kaposi’s sarcoma-associated herpesvirus, we have used a natural rodent gamma-herpesvirus experimental infection model, gamma-herpesvirus-68. In this report, we show that during long-term latent infection, naive CD8 T cells are recruited into the ongoing immune response in an epitope-specific manner. When virus reactivation is induced in vivo, the recruitment of CD8 T cells for some, but not all, epitopes is enhanced. The variation in recruitment is not due to differences in epitope presentation. We also show that CD8 T cells that are newly stimulated during reactivation are functionally impaired compared with acutely stimulated cells in terms of cytokine production. Thus, our results demonstrate unexpected complexity in the response of CD8 T cells specific for different viral epitopes that were stimulated during acute infection, quiescent latency, and reactivation.
Gillard, G. O., et al. (2011). “Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes.” PLoS Pathog 7(8): e1002141. PubMed
While immunological memory has long been considered the province of T- and B-lymphocytes, it has recently been reported that innate cell populations are capable of mediating memory responses. We now show that an innate memory immune response is generated in mice following infection with vaccinia virus, a poxvirus for which no cognate germline-encoded receptor has been identified. This immune response results in viral clearance in the absence of classical adaptive T and B lymphocyte populations, and is mediated by a Thy1(+) subset of natural killer (NK) cells. We demonstrate that immune protection against infection from a lethal dose of virus can be adoptively transferred with memory hepatic Thy1(+) NK cells that were primed with live virus. Our results also indicate that, like classical immunological memory, stronger innate memory responses form in response to priming with live virus than a highly attenuated vector. These results demonstrate that a defined innate memory cell population alone can provide host protection against a lethal systemic infection through viral clearance.
Monticelli, L. A., et al. (2011). “Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus.” Nat Immunol 12(11): 1045-1054. PubMed
Innate lymphoid cells (ILCs), a heterogeneous cell population, are critical in orchestrating immunity and inflammation in the intestine, but whether ILCs influence immune responses or tissue homeostasis at other mucosal sites remains poorly characterized. Here we identify a population of lung-resident ILCs in mice and humans that expressed the alloantigen Thy-1 (CD90), interleukin 2 (IL-2) receptor a-chain (CD25), IL-7 receptor a-chain (CD127) and the IL-33 receptor subunit T1-ST2. Notably, mouse ILCs accumulated in the lung after infection with influenza virus, and depletion of ILCs resulted in loss of airway epithelial integrity, diminished lung function and impaired airway remodeling. These defects were restored by administration of the lung ILC product amphiregulin. Collectively, our results demonstrate a critical role for lung ILCs in restoring airway epithelial integrity and tissue homeostasis after infection with influenza virus.
Sonnenberg, G. F., et al. (2011). “CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut.” Immunity 34(1): 122-134. PubMed
Fetal CD4(+) lymphoid tissue inducer (LTi) cells play a critical role in the development of lymphoid tissues. Recent studies identified that LTi cells persist in adults and are related to a heterogeneous population of innate lymphoid cells that have been implicated in inflammatory responses. However, whether LTi cells contribute to protective immunity remains poorly defined. We demonstrate that after infection with Citrobacter rodentium, CD4(+) LTi cells were a dominant source of interleukin-22 (IL-22) early during infection. Infection-induced CD4(+) LTi cell responses were IL-23 dependent, and ablation of IL-23 impaired innate immunity. Further, depletion of CD4(+) LTi cells abrogated infection-induced expression of IL-22 and antimicrobial peptides, resulting in exacerbated host mortality. LTi cells were also found to be essential for host protective immunity in lymphocyte-replete hosts. Collectively these data demonstrate that adult CD4(+) LTi cells are a critical source of IL-22 and identify a previously unrecognized function for CD4(+) LTi cells in promoting innate immunity in the intestine.