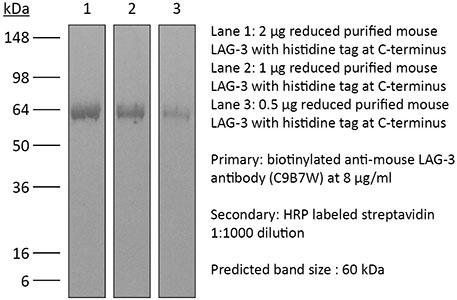
InVivoPlus anti-mouse LAG-3
| Clone | C9B7W | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalog # | BP0174 | ||||||||||
| Category | InVivoPlus Antibodies | ||||||||||
| Price |
|
The C9B7W monoclonal antibody reacts with mouse LAG-3 also known as CD223. LAG-3 is a 70 kDa type I transmembrane glycoprotein encoded by the Lag3 gene that belongs to the immunoglobulin superfamily. LAG-3 is expressed by activated T lymphocytes, NK cells, and T regulatory cells. LAG-3’s main ligand is MHC class II which it binds to with a higher affinity than even CD4 does. Upon binding LAG-3 is thought to play similar roles as CTLA-4 and PD-1 including downregulation of TCR signaling and inhibition of CD4-dependent T cell function. LAG-3 has also been demonstrated to contribute to the suppressor function of T regulatory cells. In contrast to inhibition, LAG-3 has been shown to promotes immune responses by activating antigen-presenting cells. The C9B7W antibody has been reported to block the function of murine LAG-3 in vivo and in vitro but studies suggest that the antibody does not block binding of LAG-3 to MHC class II.
| Isotype | Rat IgG1, κ |
| Recommended Isotype Control(s) | InVivoPlus™ rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure™ pH 7.0 Dilution Buffer |
| Immunogen | Mouse CD223-Ig fusion protein |
| Reported Applications |
|
| Formulation |
|
| Endotoxin |
|
| Aggregation |
|
| Purity |
|
| Sterility | 0.2 μM filtered |
| Production | Purified from tissue culture supernatant in an animal free facility |
| Purification | Protein G |
| RRID | AB_10949602 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Test Results |
|
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
INVIVOPLUS ANTI-MOUSE LAG-3 (CLONE: C9B7W)
Bauche, D., et al. (2018). “LAG3(+) Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1(+) Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis.” Immunity 49(2): 342-352 e345. PubMed
Interleukin-22 (IL-22)-producing group 3 innate lymphoid cells (ILC3) maintains gut homeostasis but can also promote inflammatory bowel disease (IBD). The regulation of ILC3-dependent colitis remains to be elucidated. Here we show that Foxp3(+) regulatory T cells (Treg cells) prevented ILC3-mediated colitis in an IL-10-independent manner. Treg cells inhibited IL-23 and IL-1beta production from intestinal-resident CX3CR1(+) macrophages but not CD103(+) dendritic cells. Moreover, Treg cells restrained ILC3 production of IL-22 through suppression of CX3CR1(+) macrophage production of IL-23 and IL-1beta. This suppression was contact dependent and was mediated by latent activation gene-3 (LAG-3)-an immune checkpoint receptor-expressed on Treg cells. Engagement of LAG-3 on MHC class II drove profound immunosuppression of CX3CR1(+) tissue-resident macrophages. Our study reveals that the health of the intestinal mucosa is maintained by an axis driven by Treg cells communication with resident macrophages that withhold inflammatory stimuli required for ILC3 function.
Rouhani, S. J., et al. (2015). “Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction.” Nat Commun 6: 6771. PubMed
Lymphatic endothelial cells (LECs) directly express peripheral tissue antigens and induce CD8 T-cell deletional tolerance. LECs express MHC-II molecules, suggesting they might also tolerize CD4 T cells. We demonstrate that when beta-galactosidase (beta-gal) is expressed in LECs, beta-gal-specific CD8 T cells undergo deletion via the PD-1/PD-L1 and LAG-3/MHC-II pathways. In contrast, LECs do not present endogenous beta-gal in the context of MHC-II molecules to beta-gal-specific CD4 T cells. Lack of presentation is independent of antigen localization, as membrane-bound haemagglutinin and I-Ealpha are also not presented by MHC-II molecules. LECs express invariant chain and cathepsin L, but not H2-M, suggesting that they cannot load endogenous antigenic peptides onto MHC-II molecules. Importantly, LECs transfer beta-gal to dendritic cells, which subsequently present it to induce CD4 T-cell anergy. Therefore, LECs serve as an antigen reservoir for CD4 T-cell tolerance, and MHC-II molecules on LECs are used to induce CD8 T-cell tolerance via LAG-3.
Durham, N. M., et al. (2014). “Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo.” PLoS One 9(11): e109080. PubMed
Lymphocyte Activation Gene – 3 (LAG-3) is an immune checkpoint molecule that regulates both T-cell activation and homeostasis. However, the molecular mechanisms underlying LAG-3’s function are generally unknown. Using a model in which LAG-3 blockade or absence reliably augmented homeostatic proliferation in vivo, we found that IL-2 and STAT5 are critical for LAG-3 function. Similarly, LAG-3 blockade was ineffective in the absence of regulatory T-cells (Treg), suggesting an important role for LAG-3 in either the responsiveness of conventional T-cells (Tconv) to regulation, or a relative defect in the ability of LAG-3 KO regulatory T-cells (Treg) to suppress the proliferation of Tconv. In this model, LAG-3 KO Treg suppressed proliferation in a manner fairly similar to wild-type (WT) Treg, but LAG-3 KO Tconv were relatively resistant to suppression. Further studies also identified a role for LAG-3 in the induction/expansion of Treg. Finally, we found that LAG-3 blockade (or knockout) led to a relative skewing of naive CD4 T-cells toward a TH1 phenotype both in vitro and in in vivo. Together, these data suggest that LAG-3 expression on Tconv cells makes them more susceptible to Treg based suppression, and also regulates the development of a TH1 T-cell response.
Erickson, J. J., et al. (2014). “Programmed death-1 impairs secondary effector lung CD8(+) T cells during respiratory virus reinfection.” J Immunol 193(10): 5108-5117. PubMed
Reinfections with respiratory viruses are common and cause significant clinical illness, yet precise mechanisms governing this susceptibility are ill defined. Lung Ag-specific CD8(+) T cells (T(CD8)) are impaired during acute viral lower respiratory infection by the inhibitory receptor programmed death-1 (PD-1). To determine whether PD-1 contributes to recurrent infection, we first established a model of reinfection by challenging B cell-deficient mice with human metapneumovirus (HMPV) several weeks after primary infection, and found that HMPV replicated to high titers in the lungs. A robust secondary effector lung TCD8 response was generated during reinfection, but these cells were more impaired and more highly expressed the inhibitory receptors PD-1, LAG-3, and 2B4 than primary T(CD8). In vitro blockade demonstrated that PD-1 was the dominant inhibitory receptor early after reinfection. In vivo therapeutic PD-1 blockade during HMPV reinfection restored lung T(CD8) effector functions (i.e., degranulation and cytokine production) and enhanced viral clearance. PD-1 also limited the protective efficacy of HMPV epitope-specific peptide vaccination and impaired lung T(CD8) during heterotypic influenza virus challenge infection. Our results indicate that PD-1 signaling may contribute to respiratory virus reinfection and evasion of vaccine-elicited immune responses. These results have important implications for the design of effective vaccines against respiratory viruses.
McGray, A. J., et al. (2014). “Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor.” Mol Ther 22(1): 206-218. PubMed
Despite clear evidence of immunogenicity, cancer vaccines only provide a modest clinical benefit. To evaluate the mechanisms that limit tumor regression following vaccination, we have investigated the weak efficacy of a highly immunogenic experimental vaccine using a murine melanoma model. We discovered that the tumor adapts rapidly to the immune attack instigated by tumor-specific CD8+ T cells in the first few days following vaccination, resulting in the upregulation of a complex set of biological networks, including multiple immunosuppressive processes. This rapid adaptation acts to prevent sustained local immune attack, despite continued infiltration by increasing numbers of tumor-specific T cells. Combining vaccination with adoptive transfer of tumor-specific T cells produced complete regression of the treated tumors but did not prevent the adaptive immunosuppression. In fact, the adaptive immunosuppressive pathways were more highly induced in regressing tumors, commensurate with the enhanced level of immune attack. Examination of tumor infiltrating T-cell functionality revealed that the adaptive immunosuppression leads to a progressive loss in T-cell function, even in tumors that are regressing. These novel observations that T cells produced by therapeutic intervention can instigate a rapid adaptive immunosuppressive response within the tumor have important implications for clinical implementation of immunotherapies.
Verhagen, J. and D. C. Wraith (2014). “Blockade of LFA-1 augments in vitro differentiation of antigen-induced Foxp3(+) Treg cells.” J Immunol Methods 414: 58-64. PubMed
Adoptive transfer of antigen-specific, in vitro-induced Foxp3(+) Treg (iTreg) cells protects against autoimmune disease. To generate antigen-specific iTreg cells at high purity, however, remains a challenge. Whereas polyclonal T cell stimulation with anti-CD3 and anti-CD28 antibody yields Foxp3(+) iTreg cells at a purity of 90-95%, antigen-induced iTreg cells typically do not exceed a purity of 65-75%, even in a TCR-transgenic model. In a similar vein to thymic Treg cell selection, iTreg cell differentiation is influenced not only by antigen recognition and the availability of TGF-beta but also by co-factors including costimulation and adhesion molecules. In this study, we demonstrate that blockade of the T cell integrin Leukocyte Function-associated Antigen-1 (LFA-1) during antigen-mediated iTreg cell differentiation augments Foxp3 induction, leading to approximately 90% purity of Foxp3(+) iTreg cells. This increased efficacy not only boosts the yield of Foxp3(+) iTreg cells, it also reduces contamination with activated effector T cells, thus improving the safety of adoptive transfer immunotherapy.
Goding, S. R., et al. (2013). “Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma.” J Immunol 190(9): 4899-4909. PubMed
Recurrent solid malignancies are often refractory to standard therapies. Although adoptive T cell transfer may benefit select individuals, the majority of patients succumb to their disease. To address this important clinical dilemma, we developed a mouse melanoma model in which initial regression of advanced disease was followed by tumor recurrence. During recurrence, Foxp3(+) tumor-specific CD4(+) T cells became PD-1(+) and represented >60% of the tumor-specific CD4(+) T cells in the host. Concomitantly, tumor-specific CD4(+) T effector cells showed traits of chronic exhaustion, as evidenced by their high expression of the PD-1, TIM-3, 2B4, TIGIT, and LAG-3 inhibitory molecules. Although blockade of the PD-1/PD-L1 pathway with anti-PD-L1 Abs or depletion of tumor-specific regulatory T cells (Tregs) alone failed to reverse tumor recurrence, the combination of PD-L1 blockade with tumor-specific Treg depletion effectively mediated disease regression. Furthermore, blockade with a combination of anti-PD-L1 and anti-LAG-3 Abs overcame the requirement to deplete tumor-specific Tregs. In contrast, successful treatment of primary melanoma with adoptive cell therapy required only Treg depletion or Ab therapy, underscoring the differences in the characteristics of treatment between primary and relapsing cancer. These data highlight the need for preclinical development of combined immunotherapy approaches specifically targeting recurrent disease.